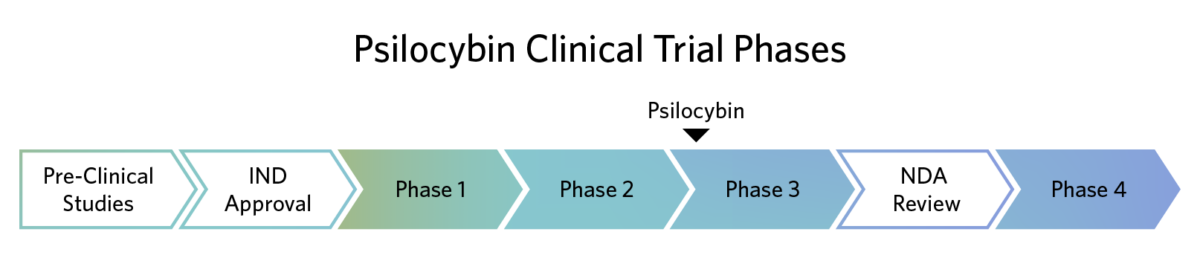
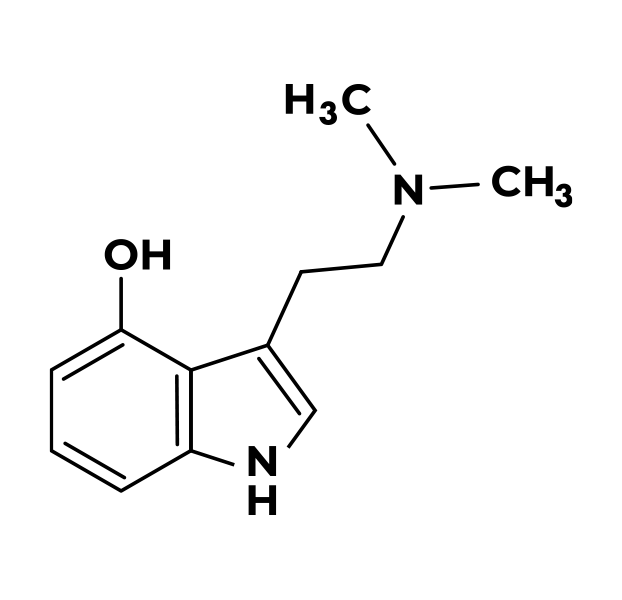
Completed Psilocybin Clinical Trials
Breakthrough Therapy Designation
The FDA describes Breakthrough Therapy designation as “a process designed to expedite the development and review of drugs that are intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint(s).“ In 2019, the FDA granted Breakthrough Therapy designation to Usona’s Investigational New Drug application for psilocybin as a potential treatment for major depressive disorder.
Expanded Access Policy
We do not offer expanded access at this time.